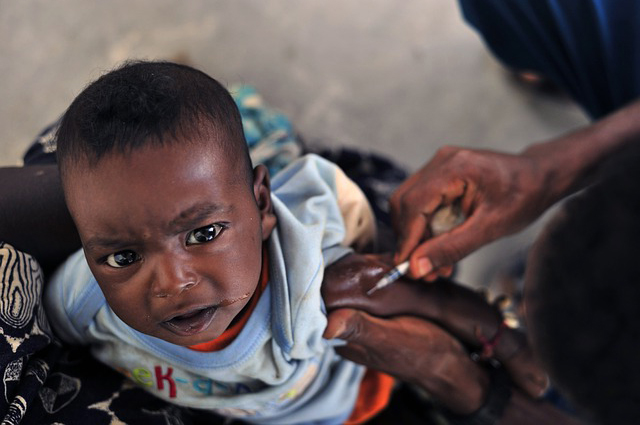
A phase 3 clinical trial of the malaria vaccine candidate RTS,S/AS01 has reported disappointing results. Three doses of the vaccine, where the first dose was administered to infants between 6 and 12 weeks of age, offered only 31% protection against detectable malaria and 37% against severe malaria. This is in contrast to an earlier trial which reported 55% protection against detectable malaria and 47% against severe malaria in children between 5 and 17 months old. The current trials, set to last until 2014, are the culmination of more than three decades of work. Despite the disappointing results, several researchers spoke out in support of continuing to develop the vaccine, reserving judgement until more data is available and citing a potential place in a suite of possible interventions – even a modest rate of protection could translate into thousands of saved lives.
Publication
RTS,S Clinical Trials Partnership. A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N Engl J Med. 2012 Dec 13;367(24):2284-95. doi: 10.1056/NEJMoa1208394. Epub 2012 Nov 9.
Coverage
- Reuters: Setback for first malaria vaccine in African trial
- New York Times: Malaria Vaccine Candidate Gives Disappointing Results
- Voice of America: Experimental Malaria Vaccine Falls Short
- Los Angeles Times: Malaria vaccine trial reports disappointing results
- SciDev.Net: Malaria vaccine trial results ‘frustrating’

